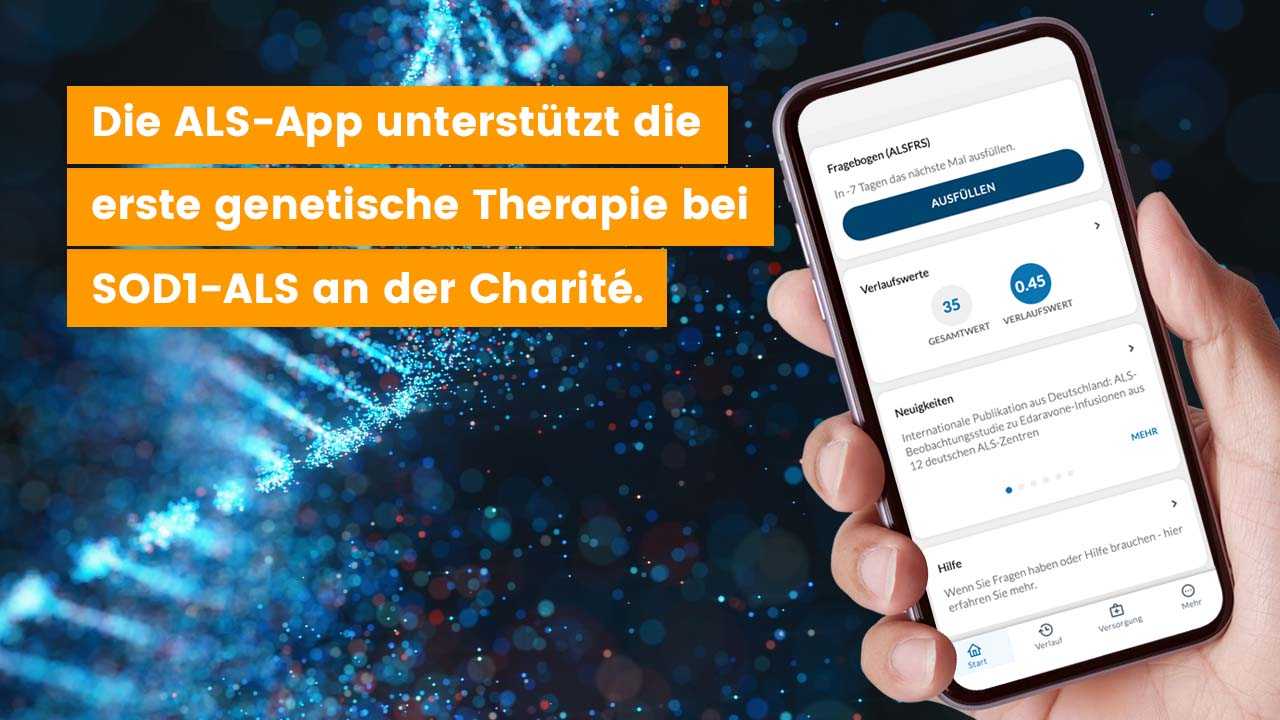
Since March 2022, two patients have been treated with the drug Tofersen at the ALS outpatient clinic of the Charité in Berlin. The documentation and monitoring of the course of treatment are supported with the ALS app.
The ALS app will be used for monitoring the Tofersen treatment first at the Charité. Other ALS outpatient clinics in Germany will follow in the coming weeks. In principle, the disease progression of all ALS patients with Tofersen in Germany can be recorded via the ALS app.
Tofersen therapy is suitable for patients who have a mutation (disease-causing genetic variant) in the SOD1 gene. Thus, this therapy option is limited to about 2% of all people affected with amyotrophic lateral sclerosis. In a clinical trial with Tofersen (VALOR trial), which was completed in November 2021, various improvements in disease progression were demonstrated (positive impact on respiratory function, significant reduction in biomarker NfL). However, a slowing of the disease based on the ALS Functional Scale (ALSFRS-R, "primary endpoint" of the study) could not be shown in the mentioned study. Despite this limitation, the German Federal Institute for Drugs and Medical Devices (BfArM) has approved the treatment of patients with SOD-1-related ALS under a hardship program.
Tofersen has not yet been approved. Therefore, research on the tolerability and efficacy of the drug is of particular importance. In the pivotal study mentioned at the beginning (VALOR study), no statistically significant effect on disease progression assessed by the ALS Functional Scale (ALSFRS-R) could be demonstrated during the 28-week treatment period. Through the hardship program, the clinical effect of Tofersen can now be documented over a longer period of time through continuous recording of the ALS Functional Scale. For this purpose, data from ALS patients with Tofersen therapy at the Charité are collected via the ALS app. Patients will be asked to rate the 12 questions of the ALS Functional Scale via the ALS app at two-week intervals.
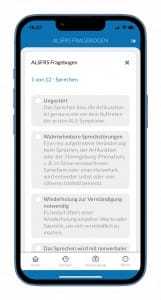
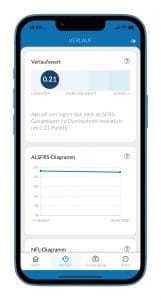
Figure: ALS app with ALSFRS history chart on the History page.
The ALSFRS data is displayed in a chart in the Ambulance Partner Portal and in the ALS app. In the ALS app, the ALSFRS chart is located on the "History" page. In the Ambulance Partner Portal, the ALSFRS diagram is displayed in the "Scales" tab.
The ALSFRS data is displayed in a chart in the Ambulance Partner Portal and in the ALS app. In the ALS app, the ALSFRS chart is located on the "History" page. In the Ambulance Partner Portal, the ALSFRS diagram is displayed in the "Scales" tab.
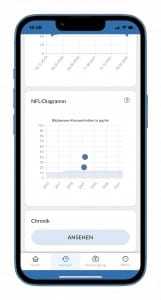
The recording of the ALS function scale is used to closely monitor whether and to what extent Tofersen can stabilize symptoms. Our research using the ALS app can contribute to a scientifically based decision on the approval of the drug. In the decision-making process by the U.S. and European regulatory agencies (FDA and EMA), our research findings on ALSFRS-R and NfL may be highly relevant.
Thank you for your interest in this project.
your APST team