People with Amyotrophic Lateral Sclerosis (ALS) suffer from slowly progressive muscle weakness (paresis), muscle wasting (atrophy) and stiffness (spasticity) of the arms and legs.
Medical exercise machines for arms and legs facilitate active (using muscular strength) or passive therapy (powered by an engine) while patients are sitting on a chair or in a wheelchair.
ALS patients can thus improve the efficiency of the muscle groups in their arms and legs whose functionality has been preserved by strengthening muscle tone, reducing spasticity and preserving joint mobility. Furthermore, regular exercise can enhance circulation to the arms and legs and reduce the risk of thrombosis and osteoarthritis. By counteracting stiffening of the joints (contractions), wear and tear of the joints (osteoarthritis) and lymphedema, pain in the joints, cartilages and muscles can be reduced or prevented.
Care Research Study on the Capturing of Exercise Machine-Based Therapy in ALS
Despite the aforementioned therapeutic benefits brought about by the use of medical exercise machines, systematically captured data on the benefits of said therapy in ALS are few and far between. The new study is designed to analyze muscle stiffness and muscle weakness symptoms as well as the patient-reported benefits of the use of exercise bikes in a real life setting.
The register study developed by APST is entitled “Digital Capturing of Treatment with THERA Exercise Machines in People with Amyotrophic Lateral Sclerosis (digitTHERA)“.
In addition to examining treatment results, the digitTHERA Care Research Study investigates the subjective perception of muscle stiffness, and muscle weakness in patients who have used a THERA Machine as part of their “exercise routine” for at least one month.
The systematic analysis of the data is intended to optimize ALS treatment with exercise bikes. The results of the study will thus contribute to developing medical guidelines and treatment recommendations for the use of exercise bikes in ALS.
What is the digitTHERA study period?
The designated study period is 24 months. The study was launched in February 2019 and will be completed in January 2021.
Where will the study be conducted
The following ALS Outpatient Departments in Germany are currently participating in the study or would like to do so:
ALS Outpatient Departments currently participating:
Alfried Krupp Krankenhaus – Ambulanz für ALS, Alfried-Krupp-Straße 21, 45131 Essen
Charité – Universitätsmedizin Berlin – Ambulanz für ALS, Augustenburger Platz 1, 13353 Berlin
Diakonissenkrankenhaus Mannheim - Klinik für Neurologie, Speyerer Straße 91 – 93, 68163
Universitätsklinikum Bergmannsheil – Ambulanz für ALS, Bürkle-de-la-Camp-Platz 1, 44789 Bochum
Universitätsklinikum Jena - Ambulanz für ALS, Erlanger Allee 101, 07747 Jena
Universitätsklinikum Leipzig – Ambulanz für ALS, Liebigstraße 20, 04103 Leipzig
Universitätsmedizin Göttingen – ALS-Ambulanz, Robert-Koch-Straße 40, 37075 Göttingen
ALS Outpatient Departments planning to take part:
Klinikum rechts der Isar der Technische Universität München – Ambulanz für Motoneuronerkrankungen, Ismaninger Straße 22, 81675 München
Medizinische Hochschule Hannover - Ambulanz für ALS, Carl-Neuberg-Straße 130625 Hannover
Universitätsklinikum Bonn - Klinik für Neurodegenerative Erkrankungen, Sigmund-Freud-Straße 25, 53127 Bonn
Universitätsklinikum Carl Gustav Carus Dresden - Ambulanz für Motoneuronerkrankungen, Fetscherstraße 74, 01307 Dresden
Universitätsklinikum Halle (Saale) - Neurologische Ambulanz, Ernst-Grube-Straße 40, 06097 Halle / Saale
Universitätsklinikum Schleswig-Holstein, Campus Kiel, Ambulanz für Muskelerkrankungen, Arnold-Heller-Straße 3, 24105 Kiel
Universitätsklinikum Ulm – Ambulanz für ALS und motorische Systemerkrankungen, Oberer Eselsberg 45, 89081 Ulm
Universitätsmedizin Rostock, ALS-Ambulanz, Gehlsheimer Straße 20, 18147 Rostock
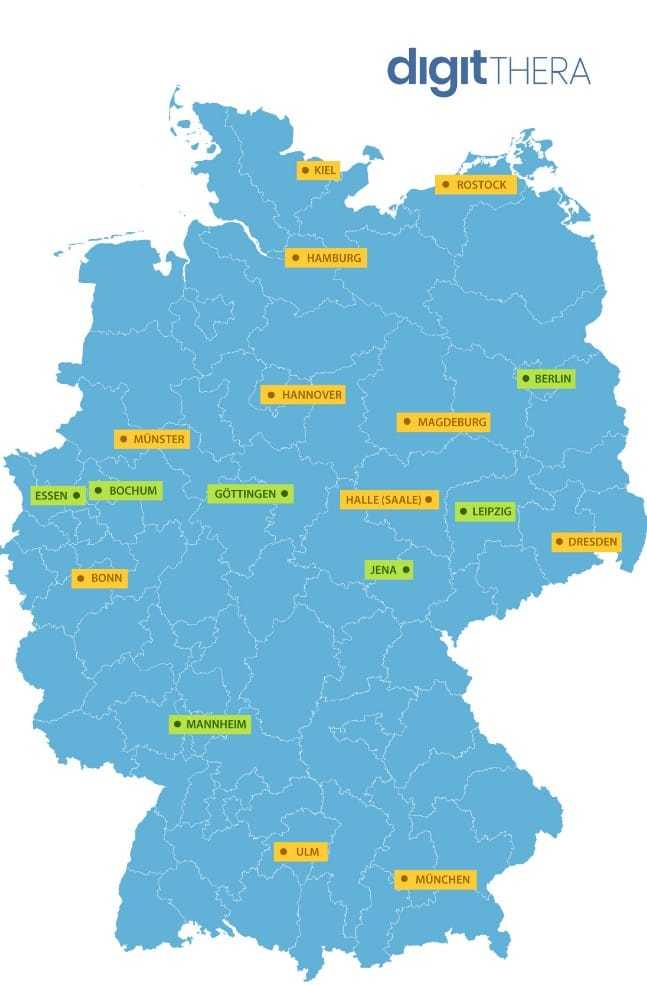
ALS Outpatient Departments that are currently participating in the digitTHERA Study (green) or are planning to take part (yellow).
What is the data capturing procedure in the digitTHERA Study?
The digitTHERA study will scientifically capture the provision of patients with a THERA bike. For this purpose, data on the use and subjective patient-reported benefit of THERA bike therapy will be collected one to six months after the THERA device has been issued to the patients.
Patients will be interviewed on their subjective experience during their scheduled visits to the study center, via the telephone or using an online questionnaire.
Who is eligible to participate?
People meeting the following criteria qualify for the study:
• ALS diagnosis
• Presence of paresis of the lower and / or upper extremities
• Successful provision of a THERA-Trainer
What is the enrolment procedure?
If you would like to take part in the digitTHERA Study, please talk to your attending physician at one of the participating ALS Outpatient Departments. You may, of course, also contact us via the telephone on +49 30 81031410 or send us an eMail at forschung@ambulanzpartner.de.
